The cardiovascular benefits of niacin (vitamin B3) were introduced in the June 1956 issue of Mayo Clinic Proceedings.1 More than 20 years later, the Framingham Heart Study touted the benefits of niacin on lipid metabolism.
The cardiovascular benefits of niacin (vitamin B3) were introduced in the June 1956 issue of Mayo Clinic Proceedings.1 More than 20 years later, the Framingham Heart Study touted the benefits of niacin on lipid metabolism.
A decade later, as the study continued, researchers labeled niacin “front-line” cardiovascular support.2 This status was further reinforced in 1988 when the National Cholesterol Education Program (NCEP) panel designated niacin a “first-line therapy” for support of specific parameters related to cardiovascular health.*3
Mechanisms of Action
Several mechanisms of action have been proposed for niacin.
Various experimental models suggest niacin can modulate lipoprotein biosynthesis in the liver, inhibit the release of free fatty acids from adipocytes, inhibit synthesis of ApoB, induce lipoprotein lipase, and help maintain the structure and function of HDL (high-density lipoprotein) by reducing the amount of apo A-1 broken down from HDL during hepatic processing.4,5 In addition, when niacin is used with resins, it can stimulate bile flow and may therefore affect lipid biosynthesis.*6
Human Trials
Since the late 1970s, studies and clinical trials, lasting from four weeks to five years with daily doses of extended-release (also known as sustained/prolonged/slow-release) niacin up to 3000 mg/day, have consistently demonstrated niacin’s efficacy and safety.6
In 2004, the ARBITER 6-HALTS trial clearly demonstrated that niacin offers targeted support of cardiovascular health.7 Final results of this trial, published in 2010, further demonstrated that niacin supports healthy carotid intima-media thickness (CIMT).8
While the supportive effect niacin has on blood lipids is well documented, there has been a recent focus by NCEP and other researchers on how niacin’s effect on HDL may influence cardiovascular events.9 More well-designed studies that address innate limitations and are carried to completion are needed.*
Why Isn’t Niacin More Widely Used?
The two common concerns are cutaneous flushing and increased liver enzymes.
Flushing
Cutaneous flushing is harmless, although it can be a nuisance. Flushing is most often seen with the use of immediate/instant-release forms of niacin and can occur with doses as low as 30 mg/day, but it is more likely to occur with the much higher doses used to support healthy blood lipids.
Flushing may last 10 to 15 minutes and rarely, but possibly, up to two hours. The proprietary wax-coated technology used in Niacin-SR tablets allows a gradual, sustained release of niacin over a seven-to-eight hour period. This delivery dramatically reduces the flushing associated with immediate-release forms.
Adherence to a regimen with the special wax-coated form of niacin, as found in Rev Niacin, ranged from 88-97% in four human clinical trials.10,11 Flushing, itching, tingling, and upper gastrointestinal side effects were minimal, but did increase when dosing was increased to 2000 mg/day.
It is important to note that Niacin-SR should not be confused with “no-flush” niacin, which is inositol hexanicotinate (IHN), a supplement that does not contain any free niacin and may not be as supportive of cardiovascular health as those providing nicotinic acid.*12,13
There are several things you can do to prevent or reduce the flushing associated with Niacin. Be sure to read our post on this issue.
Elevated Liver Enzymes
The second concern is in regards to liver enzyme elevation. This issue was first elucidated by the results of McKenney’s study, published in 1994 in the Journal of the American Medical Association (JAMA), wherein subjects received 3000 mg/day of niacin over an extended period of time.14
In April 2004, McKenney retracted his earlier warnings about the harmful effects of niacin and publicly supported its unique benefits.15 Although they generally do not enter an unhealthy range, liver enzymes may increase when initiating niacin therapy, especially in amounts greater than 1000 mg/day. Enzyme levels return to normal promptly after cessation of niacin.*15
Directions
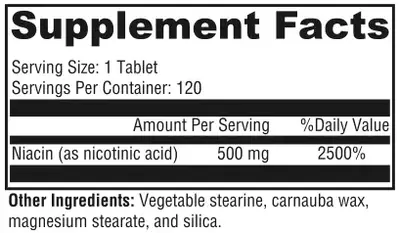
- Niacin-SR contains 500mg of niacin.
- Start with 1 tablet daily at bedtime
- If you are doing well in 3-4 weeks then increase to 1 tablet twice daily. You could take both of them at the same time before bed if that works better for you.
- If you have flushing or this seems to be going up too fast then simply cut the tablet in half (it is scored) and take 1-1/2 tablets daily.
- Try to increase again in 3-4 weeks.
- The goal is often to get from 1-2 grams of Niacin-SR each day (2-4 tablets).
![]()
References
- Parsons WB Jr, Achor RW, Berge KG,et al. Changes in concentration of blood lipids following prolonged administration of large doses of nicotinic acid to persons with hypercholesterolemia: preliminary observations. Mayo Clin Proc. 1956 Jun 27;31(13):377-90. [PMID: 13336128]
- Kannel WB. Recent findings from the Framingham study—I. Med Times. 1978 Apr;106(4):23-27. [PMID: 642745]
- Hulley SB. The US National Cholesterol Education Program. Adult treatment guidelines. Drugs. 1988;36 Suppl 3:100-04. [PMID: 3254822]
- Morgan JM, Carey CM, Lincoff A, et al. The effects of niacin on lipoprotein subclass distribution. Prev Cardiol. 2004 Fall;7(4):182-7; quiz 188. [PMID: 8424822]
- Holland RE, Rahman K, Morris AI, et al. Effect of niacin on biliary lipid output in the rat. Biochem Pharmacol. 1993 Jan 7;45(1):43-49. [PMID: 8424822]
- Niacin. [http://www.naturalstandard.com/naturalstandard/monographs/monoframeset.asp?monograph=/monographs/herbssupplements/ aux2-niacin.asp&patientVersion=/monographs/herbssupplements/patient-niacin](http://www.naturalstandard.com/naturalstandard/monographs/monoframeset.asp?monograph=/monographs/herbssupplements/ aux2-niacin.asp&patientVersion=/monographs/herbssupplements/patient-niacin). Accessed August 19, 2010. (Requires login)
- Taylor AJ, Sullenberger LE, Lee HJ, et al. Biology for the investigation of the treatment effects of reducing cholesterol (ARBITER) 2: a double-blind, placebo- controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004 Dec 7;110(23):3512-17. [PMID: 15537681]
- Villines TC, Stanek EJ, Devine PJ, et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J Am Coll Cardiol. 2010 Jun 15;55(24):2721-67. [PMID: 20399059]
- Michos ED, Sibley CT, Baer JT, et al. Niacin and statin combination therapy for atherosclerosis regression and prevention of cardiovascular disease events: reconciling the AIM-HIGH (Atherothrombosis intervention in metabolic syndrome with low hdl/high triglycerides: Impact on global health outcomes) trial with previous surrogate endpoint trials. J Am Coll Cardiol. 2012 Apr 4. [Epub ahead of print] [PMID: 22520249]
- Keenan JM, Fontaine PL, Wenz JB, et al. Niacin revisited. A randomized, controlled trial of wax-matrix sustained-release niacin in hypercholesterolemia. Arch Intern Med. 1991 Jul;151(7):1424-32. [PMID: 2064495]
- Aronov DM, Keenan JM, Akhmedzhanov NM, et al. Clinical trial of wax-matrix sustained-release niacin in a Russian population with hypercholesterolemia. Arch Fam Med. 1996 Nov-Dec;5(10):567-75. [PMID: 8930228]
- Backes JM, Padley RJ, Moriarty PM. Important considerations for treatment with dietary supplement versus prescription niacin products. Postgrad Med. 2011 Mar;123(2):70-83. Review. [PMID: 21474895]
- Norris RB. “Flush-free niacin”: dietary supplement may be “benefit-free”. Prev Cardiol. 2006 Winter;9(1):64-65. [PMID: 16407706]
- McKenney JM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994 Mar 2;271(9):672-77. [PMID: 8309029]
- McKenney JM. Pharmacologic options for aggressive low-density lipoprotein cholesterol lowering: benefits versus risks. Am J Cardiol. 2005 Aug 22;96(4A):60E- 66E. Review. [PMID: 16098846]